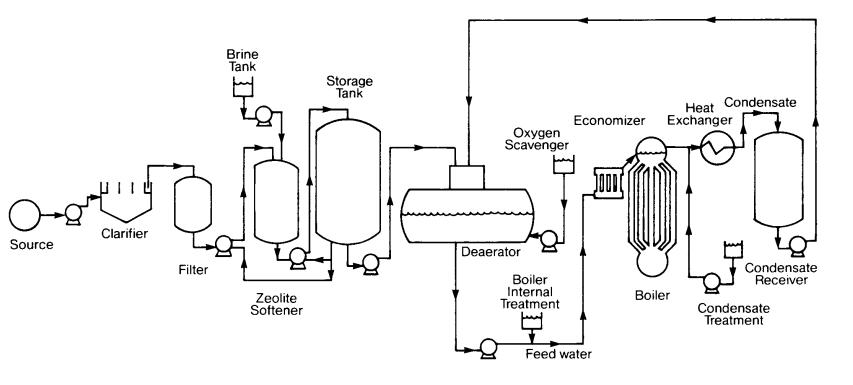
The efficient and reliable operation of industrial boilers relies heavily on proper water treatment. Boiler feedwater, the water used to supply boilers, plays a vital role in ensuring the longevity and performance of these essential equipment. Without adequate treatment, impurities in feedwater can lead to corrosion, scale formation, and reduced heat transfer efficiency. In this article, we will explore the importance of water treatment in boiler feed and delve into the various methods employed to maintain optimal water quality.
August 31, 2023

Importance of Water Treatment in Boiler Feed
Water treatment plays a crucial role in ensuring the efficient and reliable operation of boiler feed systems. Proper water treatment is essential for preventing scale formation, reducing corrosion, and minimizing sludge and sediment buildup in boilers.
One of the primary reasons for implementing water treatment in boiler feed is to prevent scale formation. When water is heated in a boiler, impurities such as calcium and magnesium precipitate out of the water and form hard deposits on heat transfer surfaces. This scale not only reduces heat transfer efficiency but also leads to overheating and equipment failure. By treating the feedwater, these impurities can be removed or controlled, preventing scale formation and maintaining optimal boiler performance.
Another critical aspect of water treatment is reducing corrosion. Corrosion occurs when metal surfaces come into contact with corrosive substances present in the water. In boilers, high temperatures and pressures accelerate corrosion rates, leading to equipment degradation and potential leaks. By removing dissolved oxygen and other corrosive elements from the feedwater through proper treatment methods, corrosion can be minimized, extending the lifespan of boiler components.
Furthermore, effective water treatment helps in minimizing sludge and sediment buildup within boilers. Sludge refers to solid particles that settle at the bottom of the boiler due to suspended impurities present in the feedwater. Over time, this sludge can accumulate and impair heat transfer efficiency while promoting localized corrosion. Through filtration processes like sedimentation or centrifugation, suspended solids can be removed before they reach the boiler system.
To achieve optimal water quality for boiler feed applications, several purification processes are commonly employed:
Filtration
Filtration involves passing water through various media or filters to remove suspended solids, sediments, and larger particles. This step helps improve overall water clarity before further treatment processes.
Types of Filtration
Several types of filters are commonly used in water treatment for boiler feed:
- Mechanical Filters: These filters physically trap suspended solids using materials like sand or fabric screens.
- Activated Carbon Filters: These filters use activated carbon to adsorb organic compounds and remove chlorine.
- High-Efficiency Particulate Air (HEPA) Filters: HEPA filters are highly effective in removing fine particulate matter, ensuring cleaner water.
Each type of filter has its own strengths and limitations, and the selection should be based on the specific requirements of the boiler feed water treatment process. Regular maintenance and replacement of filter media are essential to ensure optimal filtration performance.
Softening
Softening is an essential process in water treatment for boiler feed, as it helps to prevent the negative effects of hard water on boilers. Hard water contains high levels of dissolved minerals, primarily calcium and magnesium ions, which can lead to various operational and efficiency problems if not addressed.
Why is Softening Important?
When hard water is used as feedwater for boilers, these dissolved minerals can cause scale formation on the internal surfaces of the boiler system. Scale buildup restricts heat transfer, leading to reduced efficiency and higher energy consumption. It also increases the risk of hot spots on the metal surfaces, which can result in catastrophic failures.
By implementing a softening process, these issues can be mitigated effectively. Softening involves removing or reducing the concentration of calcium and magnesium ions, replacing them with sodium ions through a process called ion exchange. This exchange prevents scale formation and protects boiler equipment from damage.
How Does Softening Work?
The most common method employed for softening water is ion exchange, where the hardness-causing ions are exchanged with sodium ions present in a resin bed. The resin beads have a negative charge that attracts positively charged calcium and magnesium ions.
During the softening process, water flows through the resin bed, and as it comes into contact with the resin beads, calcium and magnesium ions are captured by the resin while sodium ions are released into the treated water. This ion exchange results in softened water that has a reduced concentration of hardness-causing minerals.
Benefits of Softened Water
By incorporating softening into the boiler feedwater purification process, several significant advantages can be achieved:
- Prevention of Scale Formation: Softened water reduces or eliminates scale formation inside boilers, ensuring efficient heat transfer without obstruction.
- Extended Equipment Life: The absence of scale deposits reduces wear and tear on boiler components, prolonging their lifespan.
- Improved Efficiency: Softened water allows boilers to operate at peak efficiency, maximizing energy savings and reducing operational costs.
- Reduced Maintenance: Softening minimizes the need for frequent maintenance and cleaning due to scale buildup, saving time and resources.
- Enhanced Safety: Softened water reduces the risk of hot spots and potential boiler failures associated with scale formation.
Demineralization in Water Treatment for Boiler Feed
Demineralization is a crucial step in the water treatment process for boiler feed systems. It involves the removal of mineral salts from water to prevent scale formation, corrosion, and other potential issues. By eliminating these impurities, demineralization ensures the supply of high-quality water to boilers, thereby enhancing their efficiency and longevity.
Understanding Demineralization Process
Demineralization typically involves two key methods: ion exchange and reverse osmosis.
Ion Exchange: In this process, resins are used to remove mineral ions present in water through a chemical exchange mechanism. The resin beads attract positively charged ions such as calcium (Ca2+), magnesium (Mg2+), and sodium (Na+) and replace them with hydrogen (H+) or hydroxide (OH-) ions. This helps in reducing the overall conductivity and hardness of the water.
Reverse Osmosis: Reverse osmosis utilizes a semi-permeable membrane that selectively allows water molecules to pass through while blocking dissolved minerals and contaminants. As a result, highly purified water is obtained by separating impurities from the feedwater stream.
Benefits of Demineralization
Demineralization plays a vital role in maintaining optimal boiler operation by offering several benefits:
- Scale Prevention: One of the primary objectives of demineralization is to prevent scale formationon heat transfer surfaces within boilers. Scale deposits can reduce heat transfer efficiency, leading to increased energy consumption and potential equipment failure. By removing mineral salts responsible for scaling, demineralized water helps maintain clean heat exchanger surfaces and enhances overall system performance.
- Corrosion Control: Demineralized water has low conductivity due to the absence of mineral salts. This reduces the risk of corrosionwithin boiler components like pipes, valves, and heat exchangers. Corrosion can lead to equipment damage, leaks, and decreased system reliability. Demineralization helps minimize corrosion-related issues, thus extending the lifespan of boiler equipment.
Sludge and Sediment Reduction: By removing minerals from the water supply, demineralization also helps in reducing the formation of sludge and sediment within boilers. Sludge can accumulate at the bottom of boilers or in steam distribution pipes, impeding flow rates and causing blockages. Demineralized water minimizes sludge formation, ensuring a cleaner boiler system with improved operational efficiency.
Reducing Corrosion in Water Treatment for Boiler Feed
Corrosion is a significant concern in water treatment for boiler feed systems. Corrosion can lead to equipment damage, reduced operational efficiency, and increased maintenance costs. Therefore, it is crucial to implement effective strategies to reduce corrosion and ensure the longevity of the boiler system.
One of the primary causes of corrosion in boiler feedwater is the presence of dissolved oxygen. When oxygen dissolves in water, it reacts with metal surfaces, causing corrosion. To prevent this, water treatment processes include deaeration or oxygen scavenging methods. These methods remove or reduce the concentration of dissolved oxygen in the feedwater before it enters the boiler system.
Another key factor contributing to corrosion is improper pH levels. If the pH level of the boiler feedwater is too low or too high, it can promote corrosive conditions. Acidic conditions can lead to acid attack on metallic surfaces, while alkaline conditions can result in caustic embrittlement and deposition of scale.
To mitigate corrosion caused by pH imbalance, water treatment practices include pH adjustment using neutralizing amines or other chemicals. The correct dosage of these chemicals helps maintain a balanced pH level within an acceptable range (typically between 8.5 and 9.5). Regular testing and monitoring of feedwater pH levels are essential to ensure optimal conditions for corrosion prevention.
Additionally, protective coatings and inhibitors play a vital role in reducing corrosion. These substances form a thin film on metal surfaces that acts as a barrier against corrosive elements present in the feedwater. Corrosion inhibitors work by either forming a protective layer on metal surfaces or altering the electrochemical reactions that cause corrosion.
The choice and application of inhibitors depend on factors such as water chemistry, operating conditions, and metallurgy involved. It is essential to consult with experts or chemical suppliers to determine the most suitable inhibitor for specific boiler systems.
。
Apart from managing dissolved oxygen, pH levels, and using corrosion inhibitors, other factors that contribute to corrosion include velocity of water flow and temperature. High-velocity flow can cause erosion-corrosion, which damages metal surfaces. Similarly, high temperatures can accelerate corrosion reactions. Therefore, proper system design and maintenance practices should be implemented to prevent these issues.
Minimizing Sludge and Sediment Buildup
One of the key objectives in water treatment for boiler feed is minimizing sludge and sediment buildup. Sludge refers to solid particles that settle at the bottom of the boiler, while sediment refers to suspended particles that circulate within the system. Both can cause various operational issues if left unaddressed.
When it comes to minimizing sludge and sediment buildup, there are several important considerations and best practices to follow:
- Proper Filtration: Filtration is an essential step in removing suspended solids from the boiler feedwater. Filters such as cartridge filters or multimedia filters are commonly used to capture particles down to a specific size. By effectively removing these solids, filtration helps prevent their accumulation in the boiler system, reducing the risk of sludge formation.
- Chemical Treatment: Chemical additives can be employed to prevent or minimize sludge and sediment formation. For instance, dispersants or polymeric agents can help keep solid particles suspended in the water, preventing them from settling out as sludge. Additionally, antiscalant chemicals can inhibit scale formation on heat transfer surfaces, which can contribute to sludge deposition.
- Blowdown Operations: Regular blowdown operations play a crucial role in maintaining water quality and reducing sludge accumulation. Blowdown involves removing a portion of the boiler water periodically to eliminate concentrated impurities that could lead to scale or sediment formation. By controlling blowdown frequency and volume based on water quality analysis, operators can effectively manage sludge levels within the system.
- Monitoring Water Quality Parameters: Regular testing and monitoring of water quality parameters are essential for early detection of any issues related to sludge or sediment buildup. Key parameters include total suspended solids (TSS), turbidity, conductivity, pH levels, and dissolved oxygen content. By establishing appropriate control limits for these parameters, operators can take timely corrective actions to
- Routine Equipment Maintenance: Keeping the boiler system well-maintained is crucial for minimizing sludge and sediment buildup. Regular inspection, cleaning, and maintenance of equipment such as heat exchangers, pipes, valves, and pumps can help prevent deposition of solids and ensure optimal system performance. Neglecting maintenance can lead to increased sludge accumulation, reduced heat transfer efficiency, and potential equipment failures.
By implementing these best practices for minimizing sludge and sediment buildup in water treatment for boiler feed, operators can enhance the overall efficiency and longevity of their boiler systems. This not only helps reduce operational costs but also ensures a reliable supply of steam or hot water for various industrial processes.
Remember that proper water treatment in boiler feed is vital to prevent issues such as scale formation and corrosion. The discussion on minimizing sludge and sediment buildup complements these other aspects by addressing solid particles that can compromise system performance if left unchecked.
Best Practices for Water Treatment in Boiler Feed
Proper water treatment is essential for maintaining the efficiency and longevity of a boiler system. Water treatment in boiler feed involves several best practices that help prevent scale formation, reduce corrosion, and minimize sludge and sediment buildup. By implementing these practices, operators can ensure optimal performance and extend the lifespan of their boilers.
Regular Testing and Monitoring of Water Quality Parameters
Regular testing and monitoring of water quality parameters are crucial for effective water treatment in boiler feed. This helps identify any potential issues or deviations from the desired water quality standards. Key parameters that should be monitored include pH level, conductivity, alkalinity, dissolved oxygen, hardness, and total suspended solids (TSS). By analyzing these parameters regularly, operators can take corrective actions promptly to maintain optimal water conditions.
Proper Chemical Dosage and Control
Chemical additives play a vital role in treating boiler feed water. These chemicals help prevent scale formation, control corrosion, and inhibit microbial growth. However, it is essential to ensure proper chemical dosage and control to avoid overdosing or underdosing. Overdosing can lead to excessive chemical costs and potential equipment damage while underdosing may result in insufficient protection against scaling or corrosion.
To achieve proper chemical dosing and control, operators should consider factors such as water quality analysis results, system operating conditions, manufacturer recommendations, and industry guidelines. Regular monitoring of chemical levels is necessary to maintain the desired concentration within the recommended range.
Effective Blowdown Operations
Blowdown operations are critical for removing impurities from the boiler system. Blowdown helps eliminate dissolved solids that accumulate over time due to evaporation processes. It is important to conduct blowdown operations at regular intervals based on the boiler’s design specifications or according to calculated TDS (Total Dissolved Solids) levels.
Effective blowdown operations involve proper sequencing of valves to minimize heat losses while maximizing impurity removal. The frequency and duration of blowdown should be optimized to balance the removal of impurities without wasting excessive water or energy.
Routine Equipment Maintenance
Routine equipment maintenance is essential for efficient water treatment in boiler feed. Regular inspections, cleaning, and servicing of various components such as pumps, valves, heat exchangers, and filters are crucial for ensuring their optimal performance. This helps prevent any potential issues that could compromise water quality or system efficiency.
Operators should follow manufacturer recommendations and industry best practices when it comes to equipment maintenance. Regularly checking for leaks, monitoring pressure levels, lubricating moving parts, and replacing worn-out components are some of the tasks that should be included in a comprehensive maintenance plan.
By implementing these best practices for water treatment in boiler feed, operators can significantly enhance the performance and lifespan of their boilers. Proper testing and monitoring of water quality parameters, ensuring proper chemical dosage and control, conducting effective blowdown operations, and performing routine equipment maintenance are key factors in maintaining an efficient and reliable boiler system.
Regular Testing and Monitoring of Water Quality Parameters
Testing and monitoring the water quality parameters is an essential aspect of water treatment in boiler feed. By regularly analyzing the water used for boiler feed, operators can ensure that it meets the required standards and prevent potential issues that can arise from poor water quality.
There are various parameters that need to be tested and monitored to maintain optimal water quality. Some of these parameters include:
- pH level: The pH level determines the acidity or alkalinity of the water. It is crucial to maintain a proper pH balance as it affects the efficiency of chemical treatments and prevents corrosion.
- Total Dissolved Solids (TDS): TDS refers to the total concentration of dissolved substances in the water, including minerals, salts, and other impurities. High TDS levels can lead to scale formation and reduce boiler efficiency.
- Conductivity: Conductivity measures the ability of water to conduct electricity, which is directly related to its mineral content. High conductivity indicates a high concentration of dissolved solids, requiring appropriate treatment measures.
- Chloride: Chloride levels should be closely monitored as high chloride concentrations can accelerate corrosion within the boiler system.
- Total Hardness: Hardness is caused by calcium and magnesium ions present in the water. Excessive hardness can result in scale formation on heat transfer surfaces, reducing heat transfer efficiency.
- Oxygen: Oxygen in water promotes corrosion within the boiler system. Regular testing helps identify oxygen levels and allows for proper treatment with deaerators or oxygen scavengers.
- Microbiological Contaminants: Bacteria, algae, and other microorganisms can thrive in untreated water systems, leading to fouling and biofilm formation. Regular testing helps identify any microbiological contamination for appropriate remedial actions.
To ensure accurate results, it is recommended to follow established testing procedures and use reliable testing equipment. Water samples should be collected at various points in the boiler feed system, including the make-up water source, pre-treatment equipment, and after any chemical dosing.
In addition to regular testing, continuous monitoring systems can provide real-time data on key parameters such as pH, conductivity, and dissolved oxygen levels. This allows for prompt adjustments and immediate response to any deviations from desired water quality standards.
By regularly testing and monitoring these water quality parameters, operators can detect any issues early on and take corrective actions promptly. This helps prevent scale formation, corrosion, and other problems that can negatively impact boiler efficiency and lifespan. Proper water treatment not only ensures safe operation but also contributes to energy savings and cost reduction by minimizing the need for repairs or premature replacements.
Remember that routine testing and monitoring of water quality parameters should be an integral part of a comprehensive water treatment plan for boiler feed systems.
Proper Chemical Dosage and Control
One of the key aspects in water treatment for boiler feed is ensuring proper chemical dosage and control. Implementing an effective chemical treatment program is crucial to maintain the integrity and efficiency of the boiler system.
Chemical additives are used in boiler feed water to prevent scale formation, reduce corrosion, and control microbial growth. These chemicals work by altering the properties of water, inhibiting scale-forming substances, neutralizing corrosive agents, and eliminating harmful bacteria.
The importance of proper chemical dosage and control cannot be overstated. If the chemicals are not dosed correctly or their concentrations are not properly controlled, it can lead to various issues such as:
- Inadequate Scale Control: Insufficient chemical dosage can result in scale formation on heat transfer surfaces. Scale deposits act as insulators, reducing heat transfer efficiency and leading to higher energy consumption. This can eventually result in equipment failure and costly repairs.
- Corrosion Problems: Improper chemical dosing can leave metal surfaces vulnerable to corrosion attack. Corrosion weakens the structural integrity of the system and may lead to leaks or component failures. It is essential to maintain a proper balance between corrosion inhibitors to protect the entire system from corrosion damage.
- Microbiological Growth: Inadequate control of microbial growth can lead to microbiologically influenced corrosion (MIC) which causes rapid deterioration of metal surfaces due to the presence of microorganisms like bacteria, fungi, or algae.
To ensure proper chemical dosage and control, it is recommended to follow these best practices:
Conduct a comprehensive water analysis: Regularly analyze boiler feed water samples using appropriate testing methods to determine its mineral content, pH level, alkalinity, conductivity, and other relevant parameters.
Consult with water treatment specialists: Work closely with experienced professionals who have expertise in boiler feed water treatment. They can recommend the appropriate chemical additives and dosage rates based on water analysis results and system requirements.
Use accurate chemical dosing equipment: Invest in reliable and accurate dosing equipment to ensure precise addition of chemicals. This can include chemical feed pumps, flow meters, controllers, and monitoring devices.
Establish a control strategy: Develop a control strategy that considers factors such as water quality variations, system load changes, and seasonal variations. Continuously monitor the water chemistry parameters and adjust the chemical dosages accordingly.
Regularly test and monitor: Conduct frequent testing of key water parameters to verify the effectiveness of the treatment program. Adjust the chemical dosages as per the test results to maintain optimal conditions.
Remember, proper chemical dosage and control are critical for maintaining boiler efficiency, minimizing downtime, extending equipment life, and ensuring safe operations. Neglecting this aspect could lead to costly repairs, reduced productivity, and compromised safety.
In conclusion, by implementing an effective chemical treatment program with proper dosage and control, you can significantly improve boiler feed water quality, prevent scale formation, reduce corrosion risks, minimize microbiological growth, enhance system performance, and ultimately prolong the lifespan of your boiler system.
How to Choose the Right Pure Water Treatment Equipment for Your Needs?
November 10, 2025
How to Choose the Right Pure Water Treatment Equipment for Your Needs? (by ROAGUA – Professional Water Treatment Equipment Manufacturer, China)
Turn Muddy Water into Clean Water: ROAGUA Stainless Steel Ultrafiltration Water Purifier for Africa
November 5, 2025
Clean Water for Every Village – ROAGUA Stainless Steel Ultrafiltration Water Purifier Built for Africa’s Tough Water Conditions
How Do You Select the Right 500LPH RO System?
October 14, 2025
buy right 500LPH RO System, please read this one first



