Welcome to the fascinating world of water treatment plants! Have you ever wondered what happens to the water that flows through your taps before it reaches your glass? Well, that’s where water treatment plants come into play. These marvels of engineering are amazing facilities that ensure our drinking water is safe, clean, and ready for us to enjoy. In this article, we will take a deep dive into the inner workings of water treatment plants and explore how they work their magic to provide us with high-quality water.
September 13, 2023

What is a water treatment plant?
A water treatment plant is a facility that processes raw water to make it safe for human consumption and various other purposes. Its main purpose is to remove contaminants and impurities from the water, ensuring that it meets certain standards of quality.
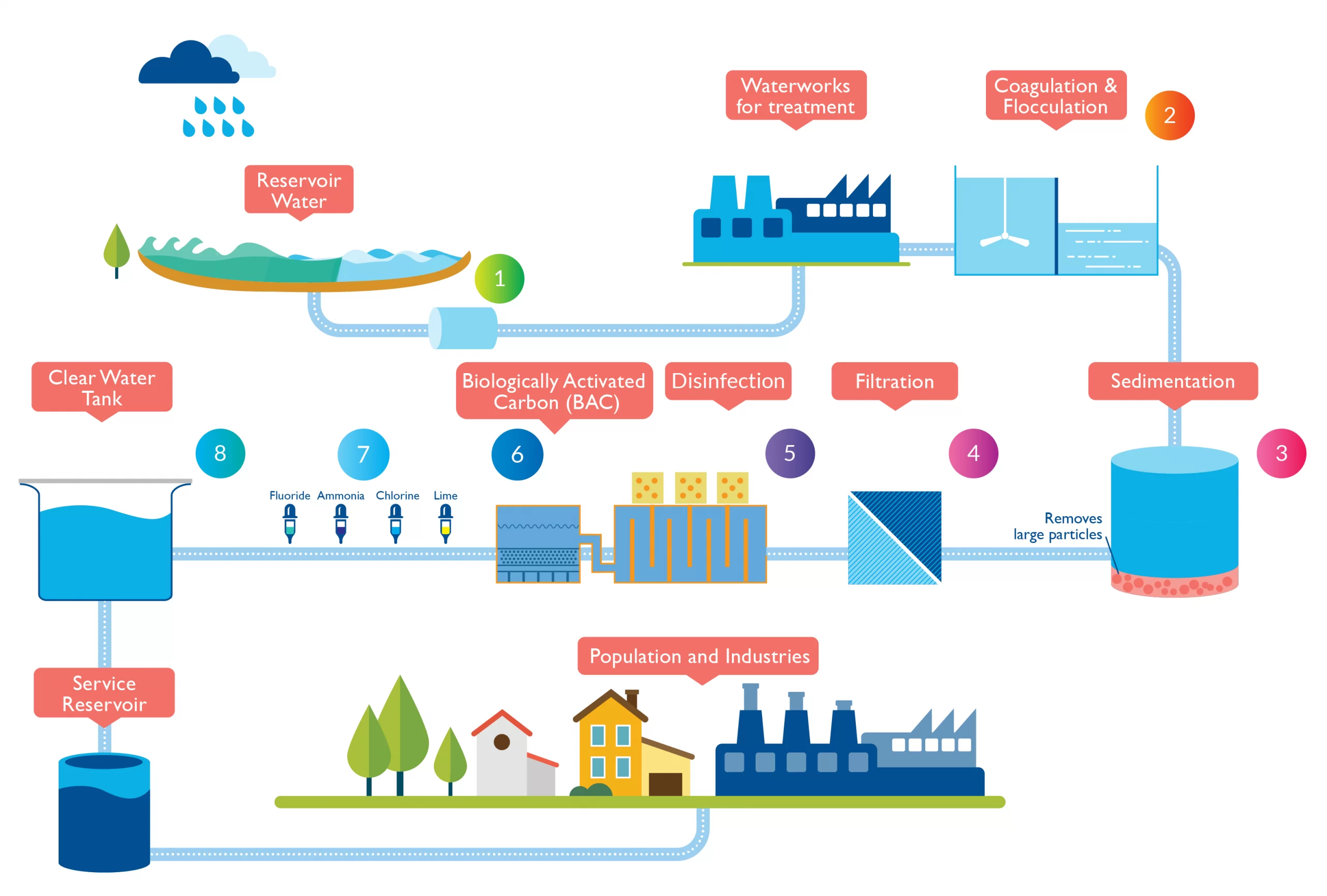
Water treatment plants consist of several components working together to achieve the desired results. These components include intake structures, where water is collected from its source, and various treatment units such as coagulation and flocculation tanks, sedimentation basins, filtration systems, disinfection units, and storage tanks.
There are different types of water treatment plants depending on the source of water and the level of treatment required. Some common types include surface water treatment plants, which treat water from rivers, lakes, or reservoirs; groundwater treatment plants that focus on treating well or borehole water; and seawater desalination plants that convert saltwater into freshwater.
Water treatment plants play a crucial role in maintaining public health by removing harmful substances from the water supply. They help eliminate contaminants such as bacteria, viruses, parasites, chemicals, heavy metals, and sediments. By doing so, they prevent the spread of diseases and ensure that clean and safe drinking water is available to communities.
Components of a water treatment plant
- Intake: This is the starting point where raw water is collected from its source, such as a river or lake. The intake structure ensures that the water is properly screened to remove large debris and prevent damage to the treatment equipment.
- Coagulation and Flocculation: In this process, chemicals called coagulants are added to the water to destabilize suspended particles. These particles then clump together to form larger particles called floc. Coagulation and flocculation help remove impurities like dirt, organic matter, and microorganisms.
- Sedimentation: After coagulation and flocculation, the water enters sedimentation basins or clarifiers where the floc settles down due to gravity. The clear water on top is then separated from the settled solids through a collection system.
- Filtration: Filtration involves passing the clarified water through various layers of filtration media such as sand, gravel, or activated carbon. These layers trap any remaining suspended particles or impurities that were not removed during sedimentation.
- Disinfection: To ensure that all harmful pathogens like bacteria, viruses, and parasites are eliminated from the treated water, disinfection is carried out. Common disinfection methods include chlorination, ozonation, or ultraviolet (UV) radiation.
- Distribution and Storage: Once treated and disinfected, the purified water is stored in reservoirs before being distributed through a network of pipes to homes and businesses for use.
How do water treatment plants work?
The process of water treatment involves several steps that aim to remove impurities and contaminants from the raw water. Each step is carefully designed to target specific pollutants and ensure the overall quality of the treated water.
The first step in the process is coagulation and flocculation. During this stage, chemicals known as coagulants are added to the raw water. These coagulants cause tiny particles and impurities to clump together, forming larger particles called flocs. The flocs help in the subsequent removal of suspended solids and other impurities.
After coagulation and flocculation, the next step is sedimentation. In this stage, the water is allowed to sit undisturbed in large basins or tanks. The heavier flocs settle at the bottom of these basins due to gravity, forming a layer of sludge. This sludge is later removed from the bottom of the tank through a process called sludge withdrawal.
Following sedimentation, filtration takes place. Water passes through various filtration media such as sand, gravel, or activated carbon beds. These filters trap any remaining suspended particles and impurities that were not removed during sedimentation.
Once filtration is complete, disinfection is carried out to eliminate harmful microorganisms present in the water. Common disinfection methods include chlorination, ultraviolet (UV) radiation, or ozonation. These processes effectively kill bacteria, viruses, and other pathogens that may be present in the treated water.
Finally, after disinfection comes distribution and storage. The treated water is pumped into a network of pipes that deliver it to homes, businesses, and other consumers. Water storage tanks are also used to ensure a continuous supply of water during peak demand periods or in case of emergencies.
Step 1: Coagulation and flocculation
Coagulation and flocculation are the initial steps in the water treatment process. These two processes play a crucial role in removing impurities from the water and preparing it for further treatment.
In coagulation, chemicals known as coagulants are added to the water. The most commonly used coagulant is aluminum sulfate, also known as alum. When alum is added to water, it reacts with the impurities present, such as dirt, bacteria, and other particles. This reaction causes the impurities to clump together and form larger particles called flocs.
Once coagulation has taken place, flocculation comes into play. During this stage, gentle mixing or stirring is applied to encourage the formation of larger flocs. This process allows the flocs to collide and stick together, forming even larger particles that are easier to remove.
The purpose of coagulation and flocculation is to facilitate the removal of suspended solids and other impurities from the water. By causing these impurities to clump together, they become heavier and settle more easily during subsequent treatment processes.
Step 2: Sedimentation
Sedimentation is a crucial step in the process of water treatment plants. It plays a vital role in removing suspended particles from the water, making it clearer and safer for consumption. During this stage, the water flows into large settling tanks or clarifiers, allowing the heavy particles to settle down to the bottom due to gravity.
The sedimentation process begins after coagulation and flocculation have taken place. Coagulants are added to the water to destabilize the suspended particles, causing them to clump together and form larger particles called flocs. These flocs then enter the sedimentation tanks where they settle down due to their increased weight and size.
The settling tanks are designed in such a way that they allow for a slow flow of water through them. This allows enough time for the flocs to settle at the bottom while clear water rises towards the top. The settled particles, known as sludge or sediment, are periodically removed from the bottom of these tanks.
To enhance the sedimentation process, some plants may use additional mechanisms such as inclined plates or tube settlers. These devices provide more surface area for floc settling and improve overall efficiency.
Once sedimentation is complete, most of the suspended solids have been removed from the water. However, some smaller particles may still remain. Therefore, further treatment steps like filtration and disinfection are necessary before distributing the treated water to consumers.
Step 3: Filtration
Filtration is a crucial step in the water treatment process that helps remove any remaining impurities from the water. After coagulation and sedimentation, where larger particles settle at the bottom, filtration ensures that even smaller particles and contaminants are eliminated.
During filtration, the water passes through various filtering materials, such as sand, gravel, or activated carbon. These materials act as barriers to trap suspended solids, bacteria, viruses, and other harmful substances present in the water. The filters are designed to have different levels of porosity to capture particles of varying sizes.
Sand filters are commonly used in water treatment plants. They consist of multiple layers of sand and gravel with decreasing particle size from top to bottom. As water flows through these layers, larger particles get trapped in the upper layers while smaller ones are caught deeper down.
Activated carbon filters are also employed for specific purposes. They effectively remove organic compounds that may cause taste or odor issues in drinking water. The activated carbon has a large surface area that attracts and adsorbs these compounds as water flows through it.
In addition to physical filtration methods, some plants utilize membrane filtration technologies like microfiltration or ultrafiltration. These membranes have microscopic pores that can block even smaller contaminants like bacteria and viruses from passing through.
The effectiveness of filtration depends on regular maintenance and cleaning of the filter media. Over time, accumulated particles can clog the filter bed and reduce its efficiency. Therefore, periodic backwashing or replacement of filter media is necessary to ensure optimal performance.
Step 4: Disinfection
Disinfection is a crucial step in the water treatment process that ensures the elimination of harmful microorganisms and pathogens from the water supply. It plays a vital role in safeguarding public health by preventing the transmission of waterborne diseases.
Once the water has undergone coagulation, flocculation, sedimentation, and filtration, it moves on to the disinfection stage. The primary objective of disinfection is to kill or deactivate any remaining bacteria, viruses, and parasites that may still be present in the treated water.
Various methods can be employed for disinfection in water treatment plants. The most commonly used method is chlorination, where chlorine compounds are added to the water to kill microorganisms. Chlorine is highly effective at destroying a wide range of pathogens and has been widely used for many years.
Another common method of disinfection is ultraviolet (UV) irradiation. UV light damages the DNA of microorganisms, rendering them unable to reproduce and causing their death. UV disinfection is considered environmentally friendly as it does not introduce any chemicals into the treated water.
Ozone treatment is another alternative for disinfecting water. Ozone is a powerful oxidizing agent that effectively kills microorganisms by damaging their cellular structure. It also helps remove taste and odor-causing compounds from the water.
In some cases, advanced oxidation processes (AOPs) may be employed for disinfection purposes. AOPs involve using a combination of strong oxidants such as hydrogen peroxide or ozone with UV irradiation or other catalysts to generate highly reactive hydroxyl radicals that destroy microorganisms.
After disinfection, the treated water undergoes further processes such as pH adjustment or addition of corrosion inhibitors before being distributed through pipes to consumers or stored in reservoirs for future use.
Step 5: Distribution and storage
After the water has undergone the processes of coagulation, flocculation, sedimentation, and filtration, it is now ready for distribution and storage. This final step ensures that the treated water reaches consumers in a safe and efficient manner.
Distribution involves the transportation of treated water from the water treatment plant to various locations such as homes, businesses, and institutions through a network of pipes. The distribution system is designed to deliver water reliably and continuously to meet the demands of consumers.
Storage plays a crucial role in maintaining a steady supply of treated water. Water treatment plants often have storage tanks or reservoirs where excess treated water can be stored during periods of low demand. These storage facilities help balance out fluctuations in supply and demand, ensuring that there is always an adequate supply of treated water available.
In addition to distribution and storage, it is important for water treatment plants to monitor the quality of the treated water throughout the entire process. This includes conducting regular testing for various parameters such as pH levels, disinfectant residual, and microbiological contaminants. By closely monitoring the quality of the treated water at different stages, any potential issues can be identified and addressed promptly.
Detailed Operating Instructions for Automatic Dosing Systems
December 26, 2024
Characteristics and Applications of Precision Filters
December 19, 2024



